DELIVERING A BROAD PORTFOLIO
FOR ALLERGY TESTING AND IMMUNOTHERAPY NEEDS
Stallergenes Greer has over a century of allergy immunotherapy experience and expertise. From our broad portfolio of testing extracts, which allow you to test for a wide range of allergies, to our testing devices, Stallergenes Greer can meet your testing needs.
Skin testing education is available to help you and your office staff confidently administer skin tests. Watch the Skintestor OMNITM demonstration video below, or learn more about the Stallergenes Greer Skin Testing Proficiency Program.
Please click here for Package Inserts with full Prescribing Information, including Boxed Warnings.
Choose From Two Allergy Skin Testing Devices to Meet the Needs of Your Practice and Patients

Quick and efficient multi-site testing:
- 10 test sites per applicator
- More tests in less time
- Large covered wells
- Designed to maintain extract integrity and eliminate spilling
- Choose between 20 or 40 well trays
- Gives you flexibility to customize a system that works best for your practice
- Self-loading
- Designed to be easy to fill

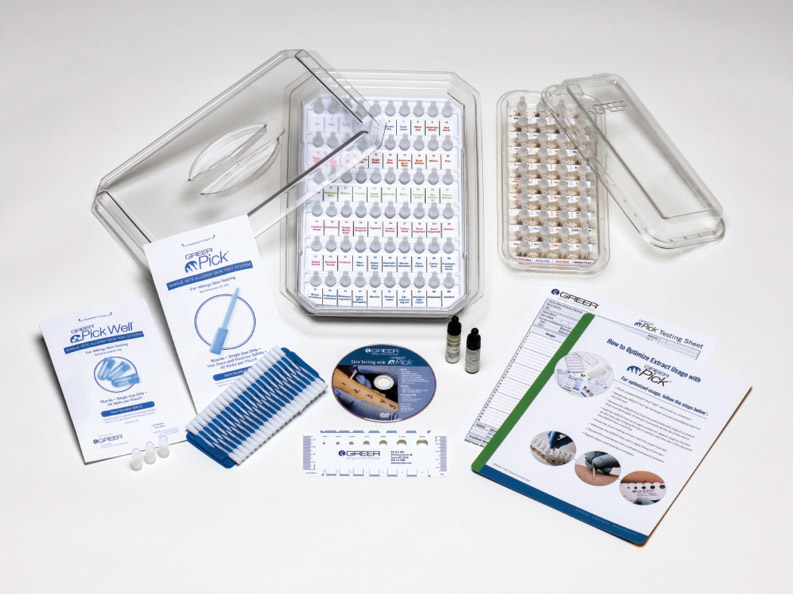
Single-use, single-site disposable skin testing system:
- Self-loading
- Designed to be easy to fill
- Large covered wells
- Designed to maintain extract integrity and eliminate spilling
- Use with 40 or 60 well trays
- Easily use a wide array of testing extracts
- Transparent well trays
- Designed to make extract levels easy to see
GREER® Pick® and Skintestor OMNITM are skin testing systems designed to administer allergenic extracts and control solutions using the prick or puncture skin testing technique.
Please see Directions for Use for GREER® Pick®.
Acquire the Skills to Use Our Skin Testing Devices With Confidence
The Stallergenes Greer Skin Testing Proficiency Program offers
THE PROFICIENCY PROGRAM IS NOW AVAILABLE ONLINE
If you’ve purchased a Skintestor OMNITM device, you can register below to request access to our online proficiency education video. A Stallergenes Greer representative may contact you if more information is needed. Everything that would be covered during a live education session is presented in a quick and engaging educational video.

If you’d like to schedule an in-person education session for the Skin Testing Proficiency Program, talk to a Stallergenes Greer Customer Care Specialist at 800.378.3906.
IMPORTANT SAFETY INFORMATION
WARNING: SEVERE ALLERGIC REACTIONS
- Allergenic extracts can cause severe life-threatening systemic reactions, including anaphylaxis.
- Do not administer allergenic extracts to patients with severe, unstable, or uncontrollable asthma.
- Observe patients in the office for at least 30 minutes following treatment. Emergency measures and personnel trained in their use must be available immediately in the event of a life-threatening reaction.
- Immunotherapy may not be suitable for patients with certain underlying medical conditions that may reduce their ability to survive a systemic allergic reaction.
- Allergenic extracts may not be suitable for patients receiving medications such as beta-blockers that may make them unresponsive to epinephrine or inhaled bronchodilators.
Do not inject intravenously. Initial dose should be based on skin test reactivity.
The most common adverse reactions include localized erythema, itching, swelling, tenderness, and pain. Systemic adverse reactions include generalized skin erythema, urticaria, pruritus, angioedema, rhinitis, wheezing, chest tightness,1 laryngeal edema, and hypotension.
INDICATIONS
Non-Standardized Allergenic Extracts: Allergenic extracts are indicated for skin test diagnosis of patients with a clinical history of allergies to one or more of the specific allergens and treatment for reduction of allergen-induced allergic symptoms confirmed by positive skin tests or in vitro testing for allergen-specific IgE antibodies. Food extracts have not been proven safe or effective in allergen immunotherapy.
Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract: Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract are indicated for skin test diagnosis of patients with a clinical history of allergy to ragweed pollen (Short Ragweed or Short and Giant Ragweed pollen), and immunotherapy for the reduction of ragweed pollen-induced allergic symptoms confirmed by positive skin test or by in vitro testing for pollen-specific IgE antibodies for Short and/or Giant Ragweed pollen.
1Standardized Cat Hair Allergenic Extract: Standardized Cat Hair Allergenic Extract is indicated for skin test diagnosis of patients with a history of allergy to cats and treatment of cat hair-induced allergic asthma, rhinitis and conjunctivitis when avoidance is not possible.
Standardized Grass Pollen Allergenic Extracts: Standardized Grass Pollen Allergenic Extracts are indicated for skin test diagnosis of patients with a clinical history of allergy to one or more of the following grass pollens: Bermuda, Kentucky Blue (June), Meadow Fescue, Orchard, Perennial Rye, Redtop, Sweet Vernal, Timothy; and immunotherapy for the reduction of grass pollen-induced allergic symptoms confirmed by positive skin test or by in vitro testing for pollen-specific IgE antibodies for Bermuda, Kentucky Blue (June), Meadow Fescue, Orchard, Perennial Rye, Redtop, Sweet Vernal, or Timothy grass pollens.
Standardized Mite Extracts: Standardized Mite Allergenic Extracts are indicated for diagnosis of skin test reactivity to dust mite allergen and treatment of mite-induced allergic asthma, rhinitis and conjunctivitis in patients that show hypersensitivity to dust mites based on clinical history, allergen exposure history, and skin test reactivity.
Please click here for Package Inserts with full Prescribing Information, including Boxed Warnings.
To report suspected adverse reactions, contact Stallergenes Greer at 1-855-274-1322 or FDA at 1-800-FDA-1088 or www.fda.gov/Safety/MedWatch.